Silver Chloride formula, also known as Silver monochloride formula or Chlorargyrite formula is explained in this article. This inorganic salt consists of a cation (Ag+) and an anion (Cl–). The chemical or molecular formula of Silver Chloride is AgCl.
It is a crystalline solid white in colour. It does not dissolve in water, alcohols, and dilute acids. It is readily soluble in ammonia, sulfuric acids, alkali cyanide, hydrochloric, and potassium bromide solution. It is naturally found as a mineral such as chlorargyrite. Commercially it can be synthesized by homogenizing aqueous solutions of sodium chloride and silver nitrate. It is a photosensitive inorganic material which is widely used in photography.
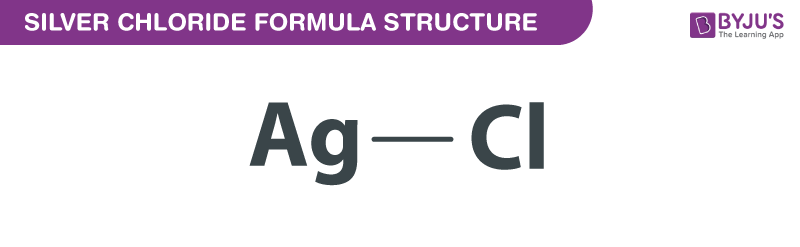
Silver Chloride Formula Structure
Properties Of Silver Chloride Formula
| Chemical formula of Silver Chloride | AgCl |
| Molecular weight of Silver Chloride | 143.32 g/mol |
| Density of Silver Chloride | 5.56 g/cm3 |
| Boiling point of Silver Chloride | 1,547 °C |
| Melting point of Silver Chloride | 455 °C |
AgCl is corrosive to metals and very harmful to the environment due to its toxicity. The toxic nature of this compound is dangerous to the wildlife in rivers and lakes. It causes irritation in the respiratory system, skin, and eyes. The light decomposes Silver chloride.
To learn more about Silver Chloride formula from the expert faculties at BYJU’S, register now!
Comments